The U.S. Food and Drug Administration (FDA) has approved Sanofi Pasteur MSD’s Gardasil 9 (Human Papillomavirus 9-valent Vaccine, Recombinant) to prevent diseases caused by nine types of Human Papillomavirus (HPV).
Gardasil 9 is an updated version of the previously FDA-approved Gardasil, which originally targeted only four HPV types. Gardasil 9 covers five more HPV types than its predecessor and can potentially prevent an estimated 90 percent of cervical, vaginal, vulvar, and anal cancers. The vaccine is approved for use in females 9 through 26 years old and males 9 through 15 years old.
Malfeasance is when a public official violates the public trust by performing an act that is wrongful, legally unjustified, or contrary to law. Nonfeasance is the failure to act where there is a duty to act. Misfeasance is conduct that is lawful but inappropriate. Perhaps, when it comes to the recent approval of Gardasil 9 all of these apply.
10 December 2014: The FDA approved the use of a reportedly ’new and improved’ version of Gardasil, which will be marketed as Gardasil 9. According to the FDA approval letter, this action was taken without consultation with VRBPAC (the Vaccines and Related Biological Products Advisory Committee) which is responsible for reviewing and evaluating data concerning the safety, effectiveness, and appropriate use of vaccines and related biological products.
The FDA approval letter, signed by Marion Gruber, Director of Office of Vaccines Research and Review CBER, states the reason for bypassing the advice of …
We did not refer your application to the Vaccines and Related Biological Products Advisory Committee because our review of information submitted in your BLA, including the clinical study design and trial results, did not raise concerns or controversial issues which would have benefited from an advisory committee discussion.
So, the Office of Vaccines Research and Review, Center for Biologics Evaluation and Research (CBER) committee took it upon themselves to decide there were ”no concerns or controversial issues” regarding the approval of Gardasil 9?
CBER decided there was no need for VRBPAC to review or evaluate any data concerning the safety, effectiveness, and appropriate use of Merck’s proposed Gardasil 9 vaccine before making a decision to approve the nine-valent HPV vaccine. This move is particularly disturbing when one considers the worldwide controversy surrounding Gardasil’s safety, effectiveness and appropriate use.
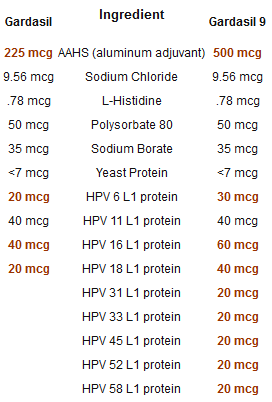
The proposed Gardasil 9 package insert and the current Gardasil package insert are a good place to start a critical examination. The table below lists the ingredients of both Gardasil and Gardasil 9. All differences from one HPV vaccine package insert to the next are highlighted.
Take a look at the first line in the chart. Aluminum is a known neurotoxin. A quick search of PubMed for ’aluminum toxicity human’ returns no less than 1652 peer-reviewed and published scientific papers on the subject. Why did Merck more than double the amount of aluminum adjuvant in Gardasil 9?What long-term health consequences are associated with the injection of 1,500 mcg of aluminum over a period of less than a year via 3 doses of Gardasil 9?
Does this risk increase if Gardasil 9 is received at the same time as another vaccine containing an aluminum adjuvant? If so, how much?
Surely the members of CBER are aware there are potential health risks resulting from aluminum exposure. Did they discuss these risks before making a decision?
Why did Merck increase the amount of HPV L1 protein for 3 of the HPV types already contained in the first version of Gardasil and not for the 4th type? Why do the amounts of these increases vary so much from one HPV type to another?
Are there any potential health risks associated with increasing the total amount of antigen (HPV L1 protein) from 120 mcg in Gardasil to 270 mcg in Gardasil 9?
There seems to be no public record of the CBER meeting, so the general public – including medical professionals who will be expected to administer this new HPV vaccine to their patients may never know whether or not these subjects were even discussed.
Bombshell #1 Serious Adverse Events
According to the FDA a serious adverse event must fit one of the following criteria: death, life-threatening, hospitalization, disability or permanent damage, congenital abnormality/birth defect, or the requirement to intervene to prevent permanent impairment.
According to the Gardasil 9 package insert, the following percentage of serious adverse events were collected during follow-up (up to 48 months):
For the first time, Merck has disclosed what may indeed be close to the true rate of serious adverse events people are suffering after the use of Gardasil and will probably continue to suffer if they consent to using Gardasil 9. The only difference would be that the rates may be higher when used in the general population because certain at-risk groups are excluded from clinical trial participation but not from vaccination programs.Keep in mind that the cost of vaccinating 100,000 people is around $30 million ($100 per injection, 3 injections). This doesn’t even begin to address the cost of treating 2,300 serious adverse events, the emotional, physical and financial expense to families and the cost to society via the lost productivity of the injured.
Bombshell #2 Systemic Autoimmune Disorders
An autoimmune disorder occurs when the body’s immune system attacks and destroys healthy body tissue by mistake. There are more than 80 types of autoimmune disorders. Many of the people diagnosed as suffering systemic autoimmune disorders after HPV vaccines were first mis-diagnosed with conversion disorder or psychosomatic illnesses. Below are the rates of “new medical conditions potentially indicative of autoimmune disorders” experienced during Merck’s Gardasil 9 clinical trials.
Bombshell #3 Pregnancy OutcomesAccording to the Gardasil 9 package insert, 1,028 women who were injected with Gardasil 9 became pregnant during the course of the clinical trials along with 991 women who had been injected with Gardasil. Overall, 14.1% of the Gardasil 9 women suffered adverse outcomes while 17.0% of the Gardasil women suffered the same fate. A total of 313 women either lost their babies to spontaneous abortion or late fetal death or gave birth to children with congenital anomalies.
This population was further broken down into those who became pregnant within 30 days of an injection and those who became pregnant more than 30 days post-injection. The charts are below.
Note: The numbers from these two charts do not add up to the total number Merck stated in the first paragraph. That is because in the ’more than 30 days’ group there were also 20 cases of congenital anomalies after Gardasil 9 and 21 cases after Gardasil.Merck stated in the package insert, “The proportions of adverse outcomes observed were consistent with pregnancy outcomes observed in the general population.”
Unless they are talking about some country other than the United States, THIS IS NOT TRUE.
According to the CDC’s latest publication on fetal mortality, the rate of spontaneous abortions and fetal deaths in the United States is 6.05/1,000 pregnancies or 0.605% – hardly 10.9%, much less 27.4%, and certainly not ’consistent with outcomes observed in the general population’ of the United States.
Do CBER officials not even go to the trouble of verifying the ’facts’ presented by vaccine manufacturers when they are ’evaluating data concerning the safety, effectiveness, and appropriate use’ of vaccines?
The vaccine was also considered to be 78 percent effective in preventing anal cancer based on the low incidence of disease caused by the five additional HPV types. The joint Sanofi Pasteur and Merck venture reported in June that the European Commission approved Gardasil for the prevention of anal precancerous lesions and anal cancers causally linked to oncogenic HPV types. The EC also granted marketing authorization for two-dose Garsadil in pediatric patients aged 9 to 13 years of age.
Please Read this Article at NaturalBlaze.com





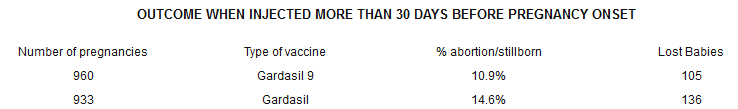



Leave a Reply